Big Question 6 - What is the Risk of Using Smokeless Products Compared to Smoking?
KEY SUMMARY POINTS
01
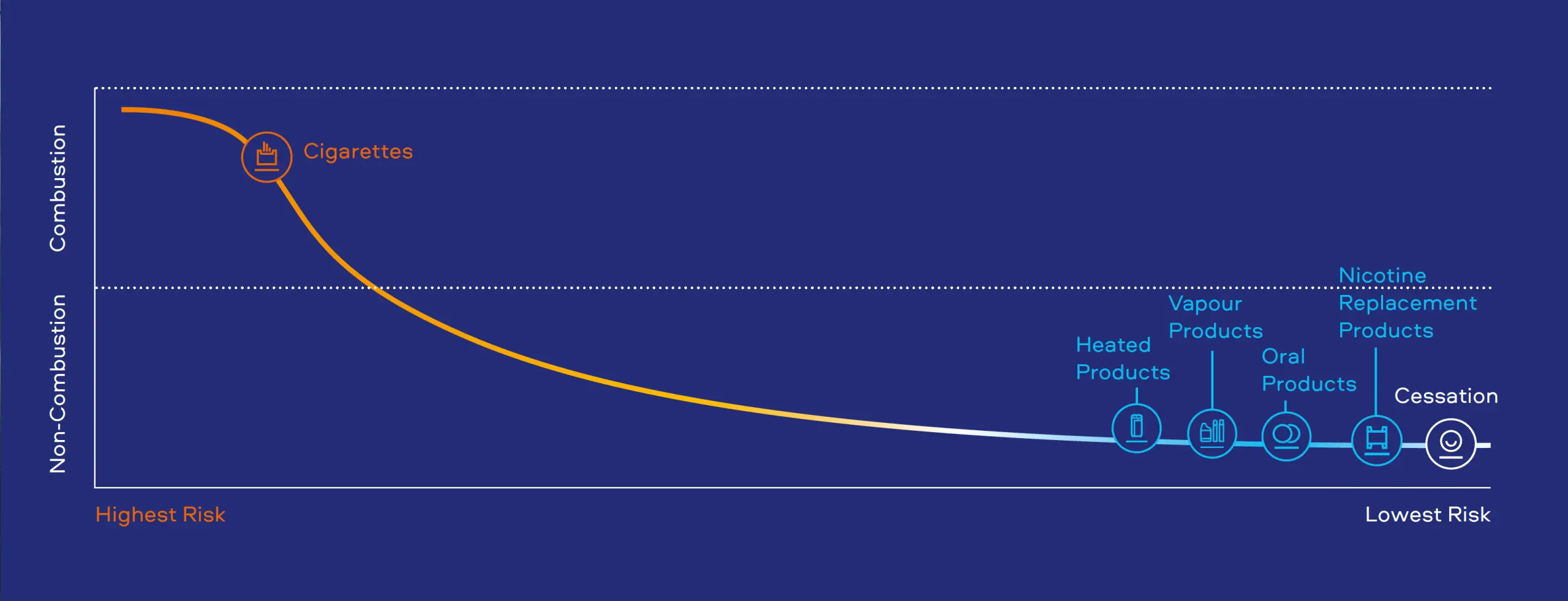
Scientific evidence confirms that Smokeless Products emit fewer and lower levels of harmful chemicals than cigarettes.
02
Fewer and lower levels of harmful chemicals in Smokeless Product emissions means lower levels of exposure for consumers, compared to smoking.
03
While Smokeless Products have a lower risk profile than smoking, they are not risk free.
"I’m fully aware of the misperceptions that are out there and aren’t consistent with the known science. We do know that e-cigarettes – as a general class – have markedly less risk than a combustible cigarette."
Dr Brian King
Director, Center for Tobacco Products, FDA [1]
The Omni™ summarises the public health impact of smoking in Chapter 3, and the scientific evidence behind our Smokeless Products in Chapter 4.
In terms of epidemiology (the study and analysis of the distribution, patterns and determinants of health and disease conditions in a defined population), we know that when people smoke cigarettes, their risk of smoking-related diseases increases. While quitting is beneficial at any age, people who quit smoking by age 40 will largely see their relative risks of smoking-related disease return to those of 'never smokers'.[2] We also know that while oral smokeless tobacco products are not risk free, people who use them have a disease risk level substantially lower than that of adult smokers.