Nicotine Replacement Therapies
We believe the best option for adult smokers is to quit. Nicotine Replacement Therapies (NRTs) are a part of the solution.
Smoking cessation
The best way for adult smokers to achieve risk reduction is to quit. Data from 2021 indicate that in the U.S., 66.5% of adults who were ever established smokers had quit smoking.[1] In Great Britain as of 2019, 62.5% of those who had ever smoked had quit.[2]
Quitting smoking can be difficult, but any smoker can quit if sufficiently motivated to do so.[3,4,5] Adult smokers use various approaches to quit, although the majority use no support (referred to as ‘cold turkey’), despite the availability of many medications and behavioural support options.
Principles of NRTs
Nicotine Replacement Therapies (NRTs), particularly nicotine gum and patches, have been widely available for more than 30 years. NRTs typically use nicotine extracted from tobacco. Designed to replace some of the nicotine obtained from smoking, they help reduce cravings and withdrawal symptoms.
Sign up for more exclusive the Omni™ content
A Timeline of Smoking Cessation Aids
1970s
First NRT prototypes developed
1978 - 84
NRT gum authorised
1996
NRT available ‘over the counter’ (OTC)
2006
Chantix/Champix authorised by EMA/FDA[7]
2009
WHO places NRT on Essential Medicines List[8]
2011
Clinical study shows one year safety usage of NRT[9]
2013
FDA extends usage period of NRT to six months[10]
2021
WHO places Varenicline and Bupropion on the Essential Medicines List[11]
2024
WHO guideline for tobacco cessation in adults published[12]
"Smoking-cessation products represent a standard [...] as a comparison product, because these products pose very few, if any, risks to health. [...] In principle, the closer the risks and exposures from the [modified risk tobacco product] are to cessation products, the more confident a regulator can be in the chances for net public health benefit"
Institute of Medicine
Scientific Standards for Studies on Modified Risk Tobacco Products, 2012 [6]
Smoking Cessation Aids and Nicotine Replacement Therapies
Gum
The first NRT made available to adult consumers. Used orally, it is intermittently chewed for around 30 minutes, as needed, to release nicotine. It delivers nicotine polacrilex which absorbs through the buccal mucosa (inner lining of the cheek) to enter the blood stream. It is available in 2-6mg of nicotine.
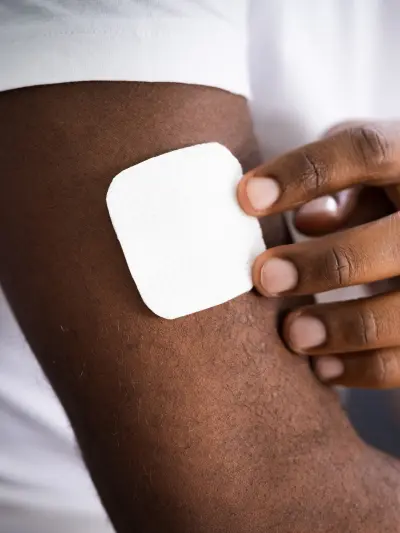
Patch
Applied to the skin, nicotine is delivered at a relatively steady rate. Patches are generally placed on the upper chest, upper arm, shoulder, back, or inner arm and worn for 16 to 24 hours. Available in a range of dosages, they can deliver between 7-21mg of nicotine over a 24-hour period.
Inhaler
Vapourised nicotine is inhaled from a plastic cartridge and mouthpiece. Frequent, short, and shallow puffs are taken. Nicotine is delivered to the oral cavity (36%) and oesophagus and stomach (36%). Lung delivery is minimal (4%). Cartridges are available in 10mg and 15mg of nicotine strengths.
Mouth spray
Sprayed directly into the mouth, nicotine is absorbed into the bloodstream through the buccal mucosa. Approximately 1mg of nicotine per spray with around 150 sprays per bottle. Nicotine mouth sprays have been shown to deliver nicotine to the blood stream faster than other NRT formulations of the same strength.
Lozenge
Placed in the mouth between the gums and cheek, the lozenge dissolves in approximately 30 minutes. Nicotine is absorbed through the buccal mucosa slowly. Available in 2mg and 4mg nicotine formulations. Nicotine lozenges can be used every 1-2 hours up to 20 pieces per day.
Nasal spray
An aerosol of nicotine is sprayed into the nose through a pump-mechanised bottle with nozzle. Nicotine is absorbed relatively rapidly compared to other NRT systems. Approximately 1mg of nicotine is delivered in two sprays, one per nostril. Users can use up to a maximum of 40 doses per day.
Micro tabs
Designed to be held under the tongue where it slowly dissolves over approximately 30 minutes. Nicotine is absorbed sublingually. Each microtab contains 2mg of nicotine.
Varenicline (Chantix/Champix)
A pill that does not contain nicotine. Varenicline is a selective nicotinic receptor partial agonist, which acts to block nicotine from binding to specific receptors in the body. This inhibits its effect. It is licensed as a prescription-only treatment for smoking cessation in the U.S. and Europe. The standard regimen is 1mg twice a day for 12 weeks.
Cytisine
A pill that does not contain nicotine. Cytisine is a selective nicotinic receptor partial agonist, which acts to block nicotine from binding to specific receptors in the body. This inhibits its effect. Cytisine is available in many countries throughout the world. The standard regimen is a 25-day course.
Bupropion (Zyban)
A pill that does not contain nicotine. Developed as an antidepressant, it is also used to aid smoking cessation. A nicotinic receptor antagonist, it blocks nicotinic acetylcholine receptors inhibiting the effect of nicotine. It is licensed for smoking cessation in the U.S., UK and Europe. The treatment course with Bupropion usually lasts from 7 to 12 weeks.
References
[2] Global Action to End Smoking, State of Smoking and Health in the United Kingdom. (Accessed: 22 July 2024)
[7] U.S. Food and Drug Administration, Drug Approval Package: Chantix (Varenicline) Tablets. 2006. (Accessed: 6 August 2008)
[8] World Health Organization, WHO Model List of Essential Medicines. 2009.
[11] World Health Organization, WHO Model List of Essential Medicines. 2021.
[12] World Health Organization, WHO clinical treatment guideline for tobacco cessation in adults. 2024.











