Unfavourable Changes and Disease
Oxidative Stress
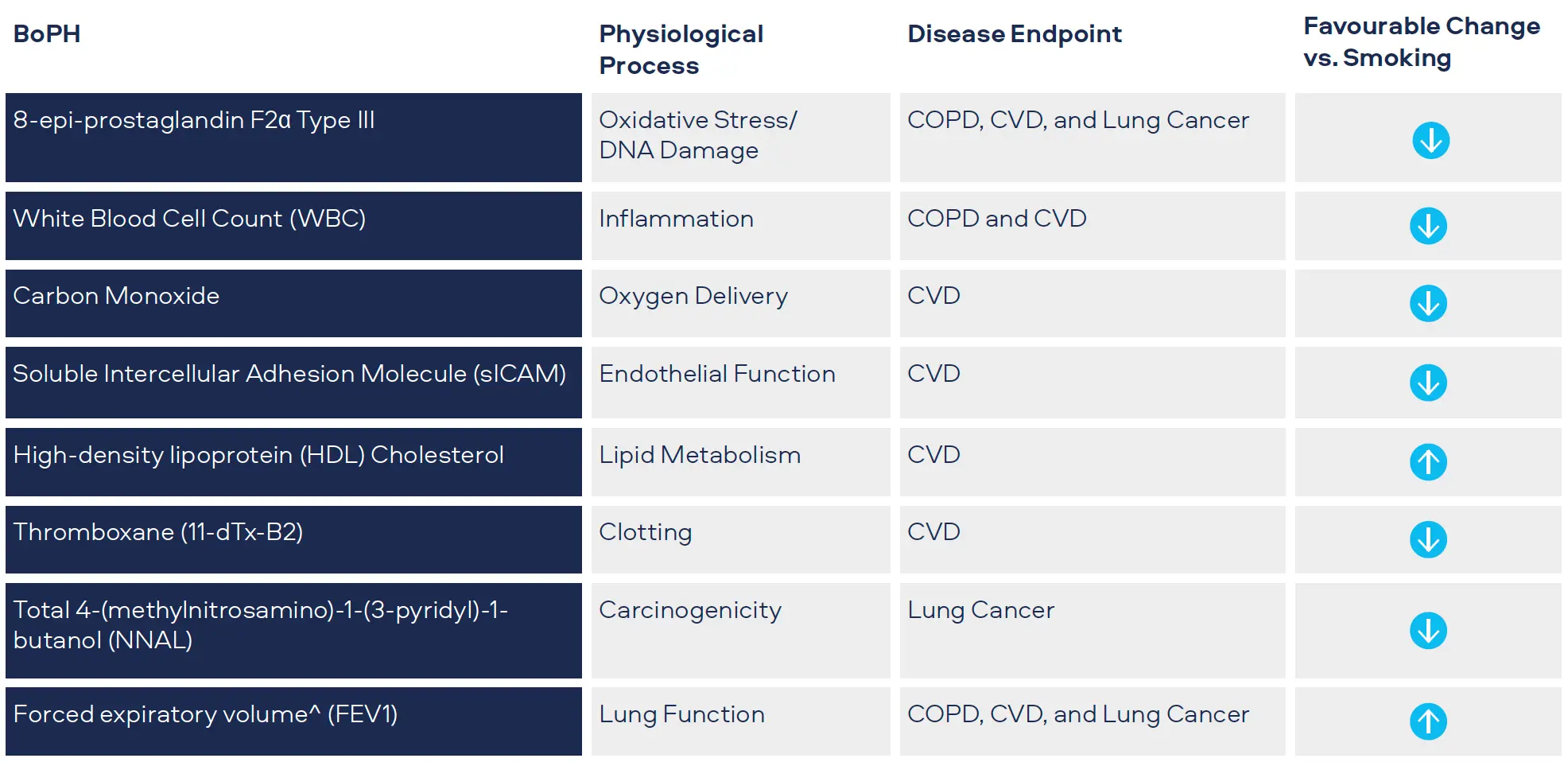
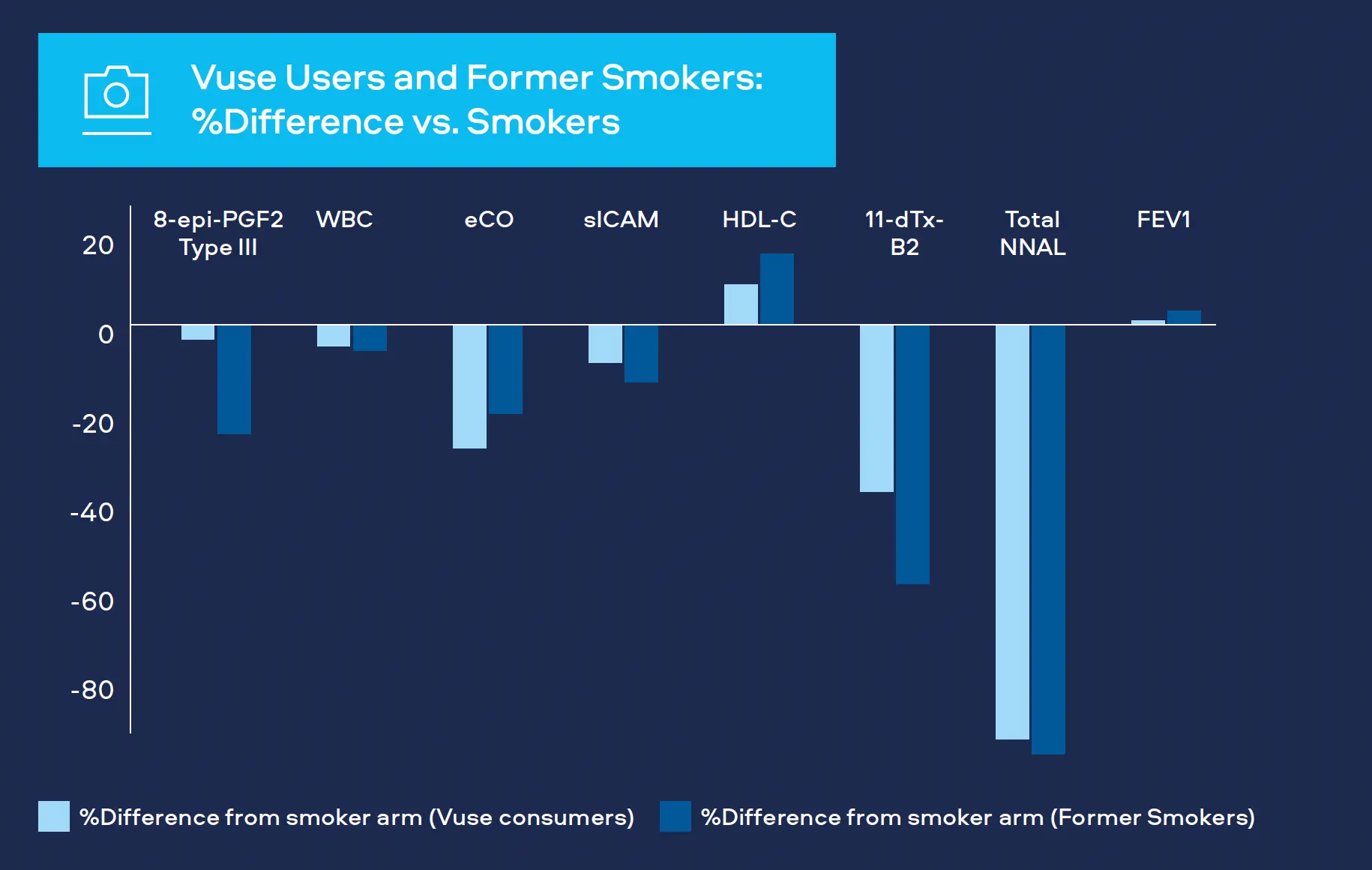
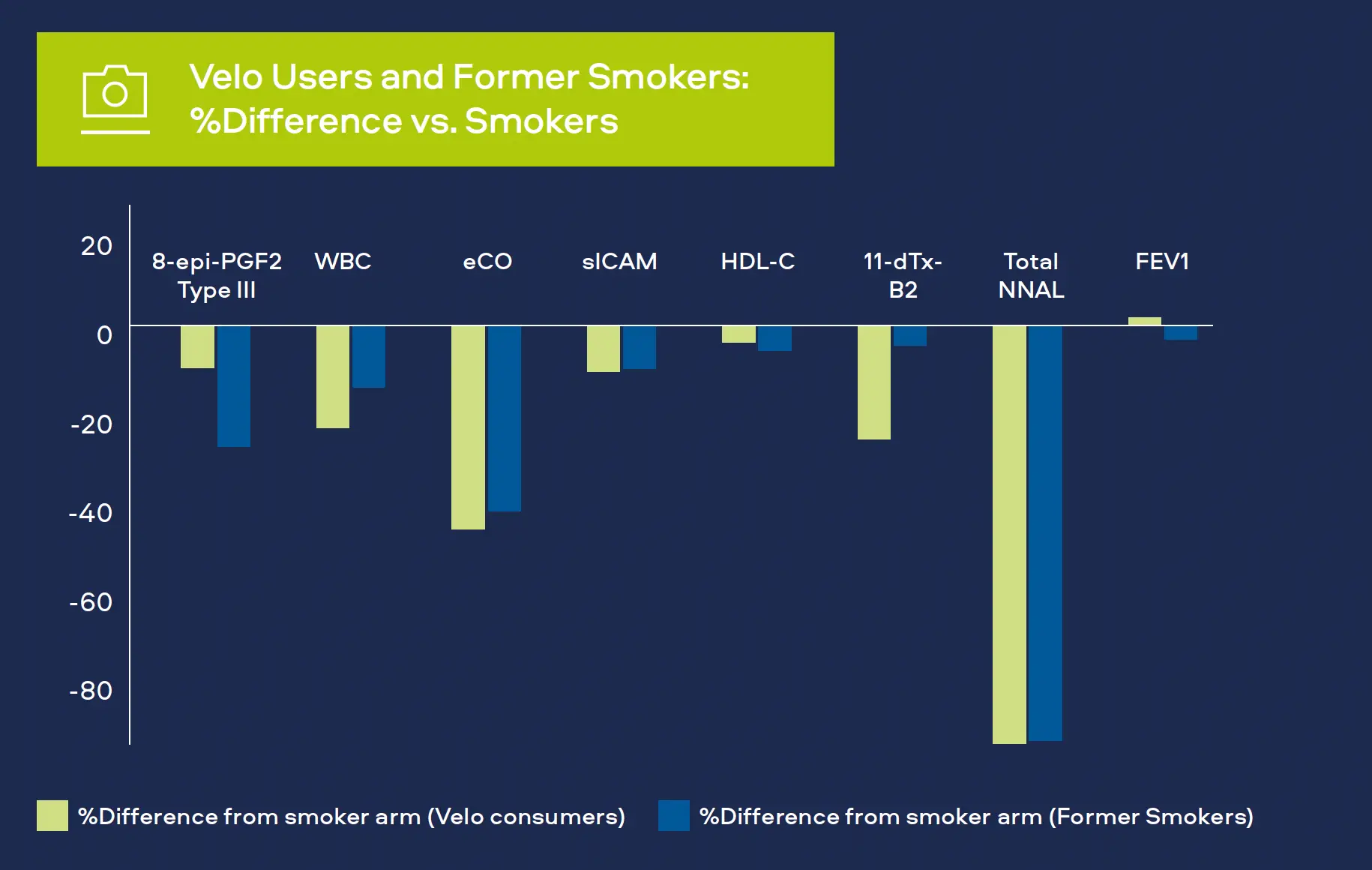
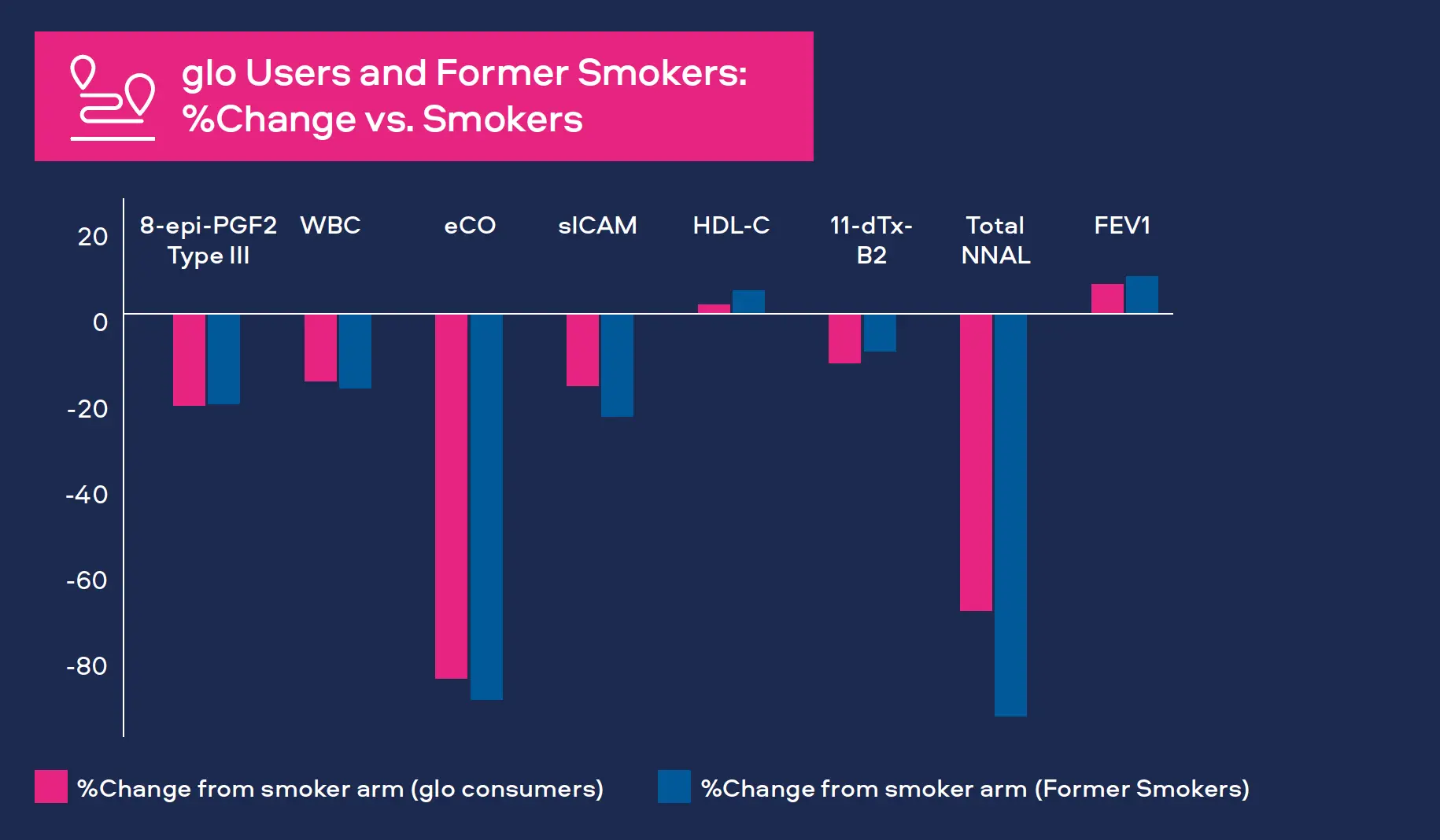
Oxidative stress is a significant factor in the development of CVD, COPD and cancer.[1,2,3] Oxidants produced as a result of cigarette smoking can cause damage to DNA and affect arteries and lung tissue. We measure 8-epiprostaglandin F2α Type III (8-epi-PGF2а Type III) as a biomarker for oxidative stress. Levels have been shown to be higher in smokers compared to never-smokers.
Inflammation
We monitor white blood cell count (WBC) as a biomarker for inflammation. White blood cells are part of the immune system and increased levels occur with bacterial and viral infections. Levels also increase with various cancers, inflammatory disorders (e.g. arthritis) and COPD, as well playing a prominent role in CVD.[4,5,6] Levels have also been shown to be higher in smokers compared to never-smokers.
CVD
We measure several biomarkers for CVD:
- Carbon monoxide
Carbon monoxide attaches to red blood cells and therefore prevents the attachment of oxygen which places stress on the heart to deliver oxygen to the body. Smoking leads to higher carbon monoxide levels in the blood compared to never-smokers. It is measured in exhaled breath (COex) or Carboxyhemoglobin the blood (COHb).[7,8]
- Soluble intercellular adhesion molecule (sICAM)
sICAM is part of the blood vessel inflammatory system. It contributes to CVD with levels in blood greater for smokers compared to never-smokers.[6]
- High-density lipoprotein (HDL) cholesterol
HDL cholesterol or ‘good cholesterol’ plays a protective role in CVD, levels tend to be lower in smokers compared to never-smokers.[6]
- Thromboxane (11-dTx-B2)
Thromboxane is a molecule produced in the blood to help it clot, generally after an injury. Levels measured in urine are greater for smokers compared to neversmokers.[6]
Lung Cancer
Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3- pyridyl)-1-butanone (NNK) has been shown to be associated with lung cancer development in smokers. Measured as total NNAL in the urine, levels in smokers are greater compared to never-smokers.[9]
Potential Detrimental Health Outcomes
Inclusive of measuring BoPH/BoBE, we assess potential detrimental health outcomes through physiological measures for all our participants such as blood pressure monitoring. We have also used spirometry, a simple test to help diagnose and monitor certain lung conditions.[10,11]