Clinical: Exposure
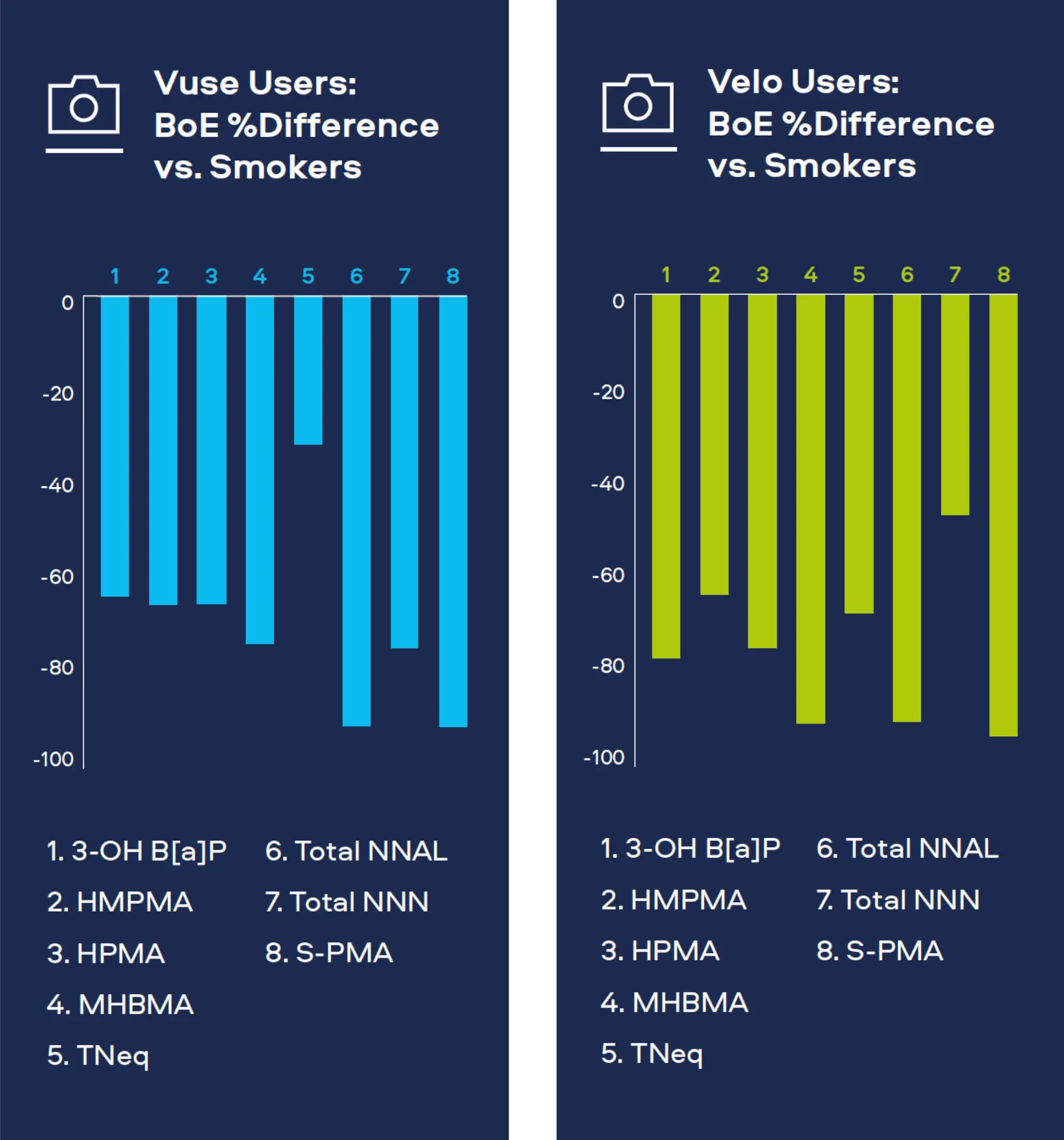
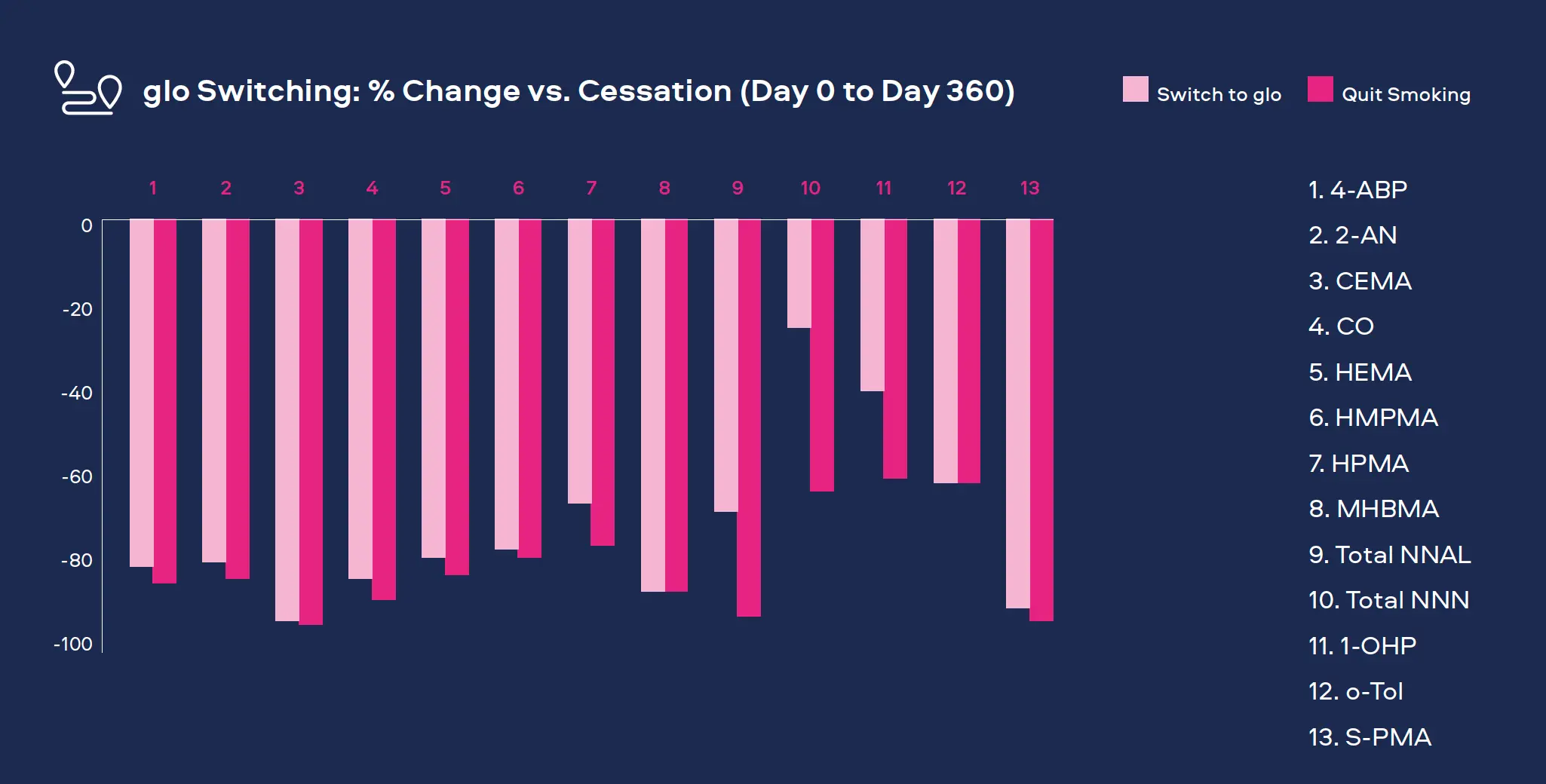
Our lab-based emission and toxicological studies have proven that our Smokeless Products deliver significantly lower levels of harmful chemicals than cigarette smoke. To understand the potential impact of these reductions on adult smokers who completely switch to our Smokeless Products, we conduct clinical studies for both exposure and potential risk.

"Adult smokers who completely switch to our Smokeless Products can greatly reduce their exposure† to a number of harmful chemicals as compared to continued smoking."
Dr Kristen Jordan
Senior Director Individual
Health Impact