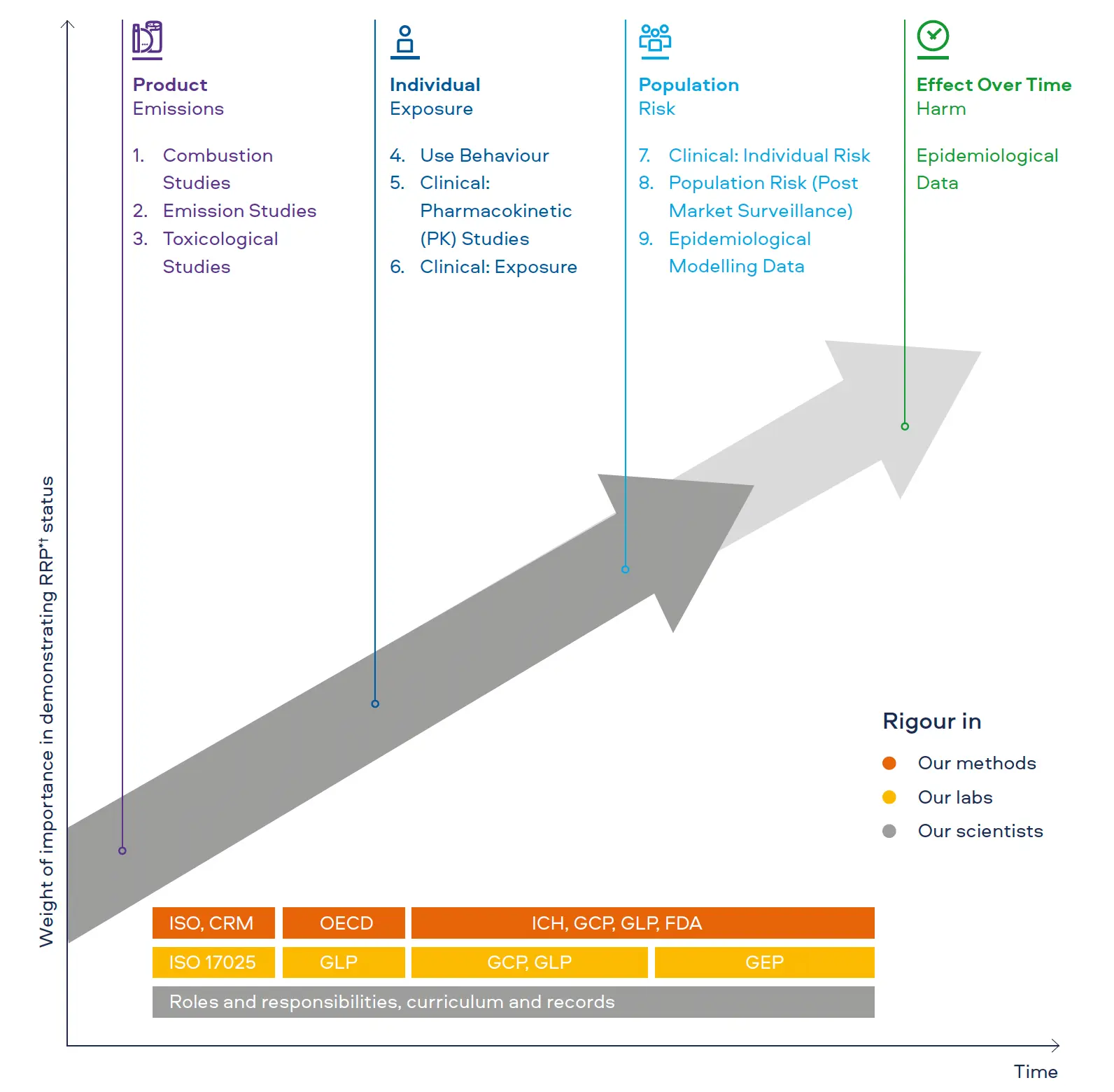
These elements cut across many dimensions; from the quality of the laboratories performing the studies and the methods utilised to generate data, to the competency of the scientists conducting the research and the systems that capture and archive these data. These elements of scientific rigour are:
Quality Systems
As a fundamental principle, the quality of scientific data is only as good as the quality of the laboratory that performs the research. For chemistry analyses such as combustion and emissions studies, our laboratories adhere to the International Organization for Standardization (ISO) standards for (1) quality management systems (ISO 9001) and (2) competence, impartiality, and consistent operation of laboratories (ISO 17025).
For toxicological assessments, studies are conducted in accordance with Good Laboratory Practice (GLP).
In clinical or observational assessments involving human subjects, studies are conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP) E6(R2). If applicable, bioanalytical procedures are conducted under GLP.
For population-level assessments such as cross-sectional surveys or post market surveillance, studies are conducted in accordance with the guidelines for Good Epidemiological Practice (GEP).
Scientific Methods
Incremental to a laboratory’s quality system, scientific data is most reliable when generated using methods that have been validated, assessed through intra-laboratory studies, and standardised.
For chemistry analyses, our data is generated with ISO methods or CORESTA (Cooperation Centre for Scientific Research Relative to Tobacco) recommended methods (CRM). If neither option exists, internally developed methods are validated and added to the laboratory’s scope of accreditation under ISO 17025.
Regulatory toxicology studies are conducted to the relevant, internationally agreed testing guidelines by the Organisation for Economic Co-operation and Development (OECD).
Methods for assessments with human subjects, at the individual or population level, are conducted in accordance with ICH E6(R2), GCP, GLP, and GEP where relevant, other applicable regulatory requirements, and/or relevant guidance for tobacco product regulatory applications (e.g. U.S. Food and Drug Administration).
Scientific Training
The rigour of our laboratories and methods means little without the strength of our most important asset, our people. That’s why initial and ongoing training of our scientists is a critical component to the science we conduct on our Smokeless Products.
Our chemists are trained to perform their assigned tasks through their education, training and experience. Technical proficiency may include, but is not limited to, initial test method training on specific work instructions, web-based technical trainings with skill or competency demonstration, formal training by an external training facilitator, refresher training, and on-the-job training administered by a subject matter expert. A training matrix is maintained as a record of employees trained to perform specific methods.
For our preclinical scientists, team members are trained in GLP, and training records are maintained for relevant standard operating procedures (SOPs).
Training for our clinical researchers and, as relevant, our epidemiological researchers, is focused on the fundamentals of ethical research, conduct of research in accordance with standards, clarity on roles and responsibilities, and ensuring the quality, accuracy, and credibility of study data. All team members are trained in GCP and, as relevant, GLP (21 CFR Part 58).
Specific roles and responsibilities for each team member are assigned and communicated via individual job descriptions and associated training curricula. This ensures that personnel possess the necessary training, skills, experience, and education to adequately perform their assigned responsibilities. All training is documented in our electronic quality management system (e-QMS).
Rigour in our 9-Step Risk Assessment Framework