U.S.: Our Vapour Products Story
Vapour Products first entered the U.S. market in 2007. Since their introduction they have evolved extensively and grown in popularity with adult prevalence rates at a high of 6.4% in 2023.[1]
AT A GLANCE
6.4%
Vapour Product prevalence has grown in the U.S. to 6.4% from 2007-2023
-7.1%
Smoking prevalence in the U.S. has decreased by 7.1% between 2012-2023
16
Vuse has one of the broadest portfolios of legal adult Vapour Products on the U.S. market with 16 Market Granted Orders

Sign up for more exclusive the Omni™ content
The growth of the vapour category in the U.S. parallels an accelerated decline of combustible cigarette smoking. Data from the Centers for Disease Control and Prevention’s (CDC) National Health Interview Survey, a U.S. nationally representative cross-sectional household interview survey, show that “current use” of combustible cigarettes by U.S. smokers (ages 18+) has declined from 18.1% in 2012 to 11% in 2023.[1] Conversely, “current use” of e-cigarettes by U.S. consumers (ages 18+) has grown from 1.9% in 2012 to 6.4% in 2023 (Figure 1).[1,2,3] In the 2019-2023 timeframe, the prevalence reduction of smoking and increase in Vapour Product use, respectively, appear linked. Applying a line of best fit would suggest that Vapour Products may be a key driver of the decline in adult smokers.
![Figure 1: Trends in Current Cigarette and E-Cigarette Use Among U.S. Consumers (18+)[1,2,3]](/gb/en/chapters/chapter-6/us-our-vapour-products-story/_jcr_content/root/container/batcom_container/batcom_container/batcom_container/batcom_image_1694507.coreimg.png/1746524329497/6-4-figure1.webp)
Figure 1: Trends in Current Cigarette and E-Cigarette Use Among U.S. Consumers (18+)[1,2,3]
Public Health Impact of Vapour vs. Smoking
The Food and Drug Administration (FDA) has acknowledged that "No tobacco product is safe. However, the health risks for different tobacco products exist on a spectrum, which is sometimes referred to as a 'continuum of risk.' Combusted, or smoked, tobacco products - such as cigarettes - are the most harmful type of tobacco product. Non-combusted products - such as e-cigarettes and other smokeless tobacco products - generally have lower health risks than cigarettes and other combustible tobacco products."[4] The National Academies of Sciences, Engineering, and Medicine also found that there "is substantial evidence that except for nicotine, under typical conditions of use, exposure to potentially toxic substances from e-cigarettes is significantly lower compared with conventional cigarettes" and "conclusive evidence that completely substituting e-cigarettes for combustible tobacco cigarettes reduces users’ exposure to numerous toxicants and carcinogens present in combustible tobacco cigarettes."[5]
In 2016, the FDA began to regulate Vapour Products as tobacco products, requiring regulatory approval for new products to be legally marketed in the U.S. and banning sales to anyone under 18 years old (later increased to 21 years old in 2019). Manufacturers are required to submit a Premarket Tobacco Product Application (PMTA). Each PMTA will need to demonstrate that the product meets all the regulatory requirements and has sufficient scientific evidence to conclude it is “appropriate for the protection of the public health” (APPH). The FDA’s thorough scientific review of all scientific evidence determines if a product is APPH. Successful applications are granted a marketing granted order (MGO) by the FDA, allowing it to be sold in the U.S.
"With more than 15 Marketing Granted Orders, Vuse has one of the broadest portfolios of authorized Vapour Products in the U.S. market."
Dr Elaine Round
Senior Vice President, U.S. Scientific & Regulatory Affairs
RAI Services Company

Vuse and the PMTA Process
Under the brand Vuse, BAT's affiliate RJ Reynolds Vapor Company launched its first Vapour Products in June 2013. Through the release of Vuse Solo, Vuse Ciro, Vuse Vibe, and Vuse Alto, we have contributed to the decline in smoking prevalence through offering adult smokers these Vapour Products.
FDA’s “deeming regulation," which deemed Smokeless Products—including Heated Products, Vapour Products and Oral Nicotine Pouches—to be under the agency’s authority and subject to the same regulatory and statutory requirements of cigarettes and other traditional tobacco products, took effect 8 August 2016. The newly applied provisions included a requirement that all “deemed” products must receive premarket authorization from the FDA to be legally marketed. Manufacturers of products on the market as of the effective date were required to submit PMTAs by 9 September 2020.
We successfully processed several PMTAs for the broad portfolio of Vuse Solo, Ciro, Vibe, and Alto Vapour Products. Through the PMTA process, we have submitted comprehensive scientific data packages, including hundreds of thousands of pages of data and thousands of scientific documents. These PMTAs were designed to help the FDA determine that the marketing of Vuse Vapour Products is appropriate for the protection of the public health.
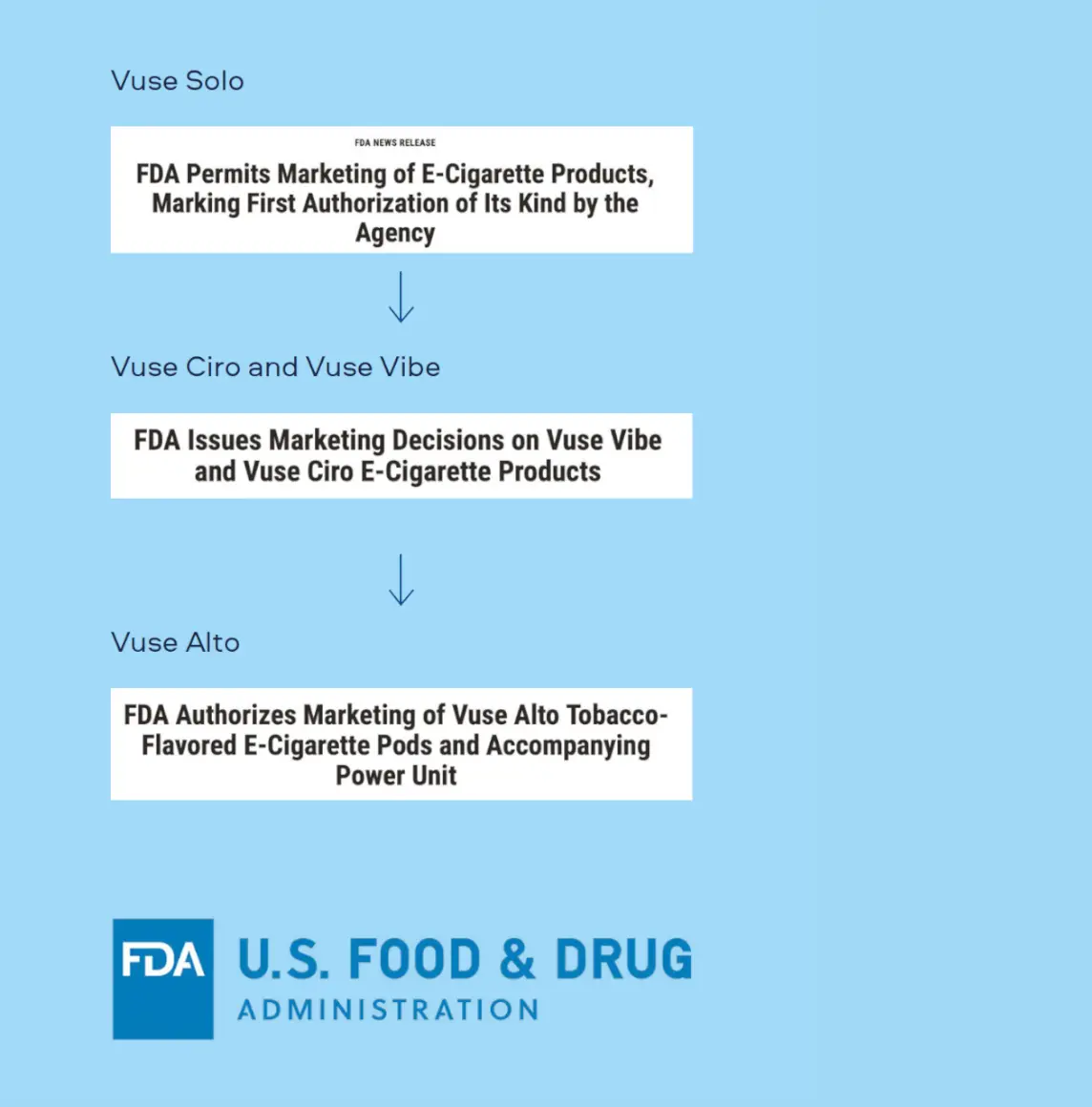
The FDA issued the first-ever Vapour Product Marketing Granted Orders (MGOs) for Vuse Solo in October 2021. Additional MGOs followed for Vuse Ciro and Vuse Vibe in May 2022. In July 2024, the FDA issued MGOs for the Vuse Alto device and its Golden Tobacco and Rich Tobacco flavoured pods. The current FDA-authorized Vuse Vapour Products represent one of the broadest portfolios of legal Vapour Products on the U.S. market with 16 MGOs.
U.S. Vapour Journey
2010
Internal research and development on an
e-cigarette product begins
June 2013
Vuse Solo launches in Colorado; expands to
Utah shortly thereafter
June 2014
National launch of Vuse Solo
2016
Launch of Vuse Ciro, Vibe, and Alto
2020
Vuse Solo, Ciro, Vibe, and Alto PMTAs are filed with the FDA
October 2021
Vuse Solo device and Original flavour cartridges receive FDA Marketing Granted Orders (MGO) - the first authorizations for Vapour Products[6]
April 2022
Vuse becomes #1 vapour brand in the U.S.[7]
May 2022
Vuse Vibe & Vuse Ciro devices and Original flavour cartridges receive FDA MGOs[8]
July 2024
Vuse Alto device and Golden and Rich Tobacco flavour pods receive FDA MGOs - Vuse Vapour Products become one of the broadest portfolios of legal Vapour Products on the U.S. market[9]
References
[1] U.S. Centers for Disease Control and Prevention, National Centers for Health Statistics, National Health Interview Survey. (Accessed: 28 August 2023)
[4] U.S. Food and Drug Administration, The Relative Risks of Tobacco Products. (Accessed: 7 August 2024)
[6] U.S. Food and Drug Administration, FDA Permits Marketing of E-Cigarette Products, Marking First Authorization of Its Kind by the Agency. 2021. (Accessed: 29 July 2024)
[7] Based on U.S. Marlin Value Share of Total Vapor (Feb. 2022 – Apr. 2022).
[8] U.S. Food and Drug Administration, FDA Issues Marketing Decisions on Vuse Vibe and Vuse Ciro E-Cigarette Products. 2022. (Accessed: 29 July 2024)
[9] U.S. Food and Drug Administration, FDA Authorizes Marketing of Vuse Alto Tobacco-Flavored E-Cigarette Pods and Accompanying Power Unit. 2024. (Accessed: 7 August 2024)











