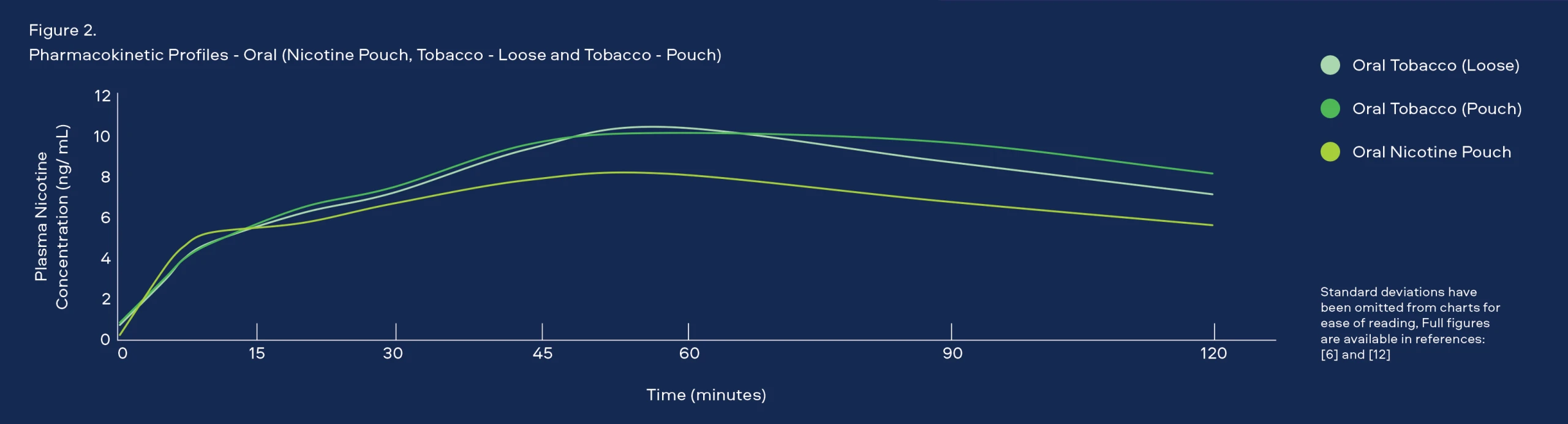
Use Behaviour and Pharmacokinetic Studies

"Our 'use behaviour' studies show that adult consumers use our Smokeless Products in a manner that will not offset the lab-measured reductions in emissions. This use behaviour gives us confidence that we should see reductions in clinical measures when we assess biomarkers of exposure and biomarkers of potential harm."
Dr Sarah Baxter-Wright
Vice President, U.S. Life Sciences, RAI Services Company
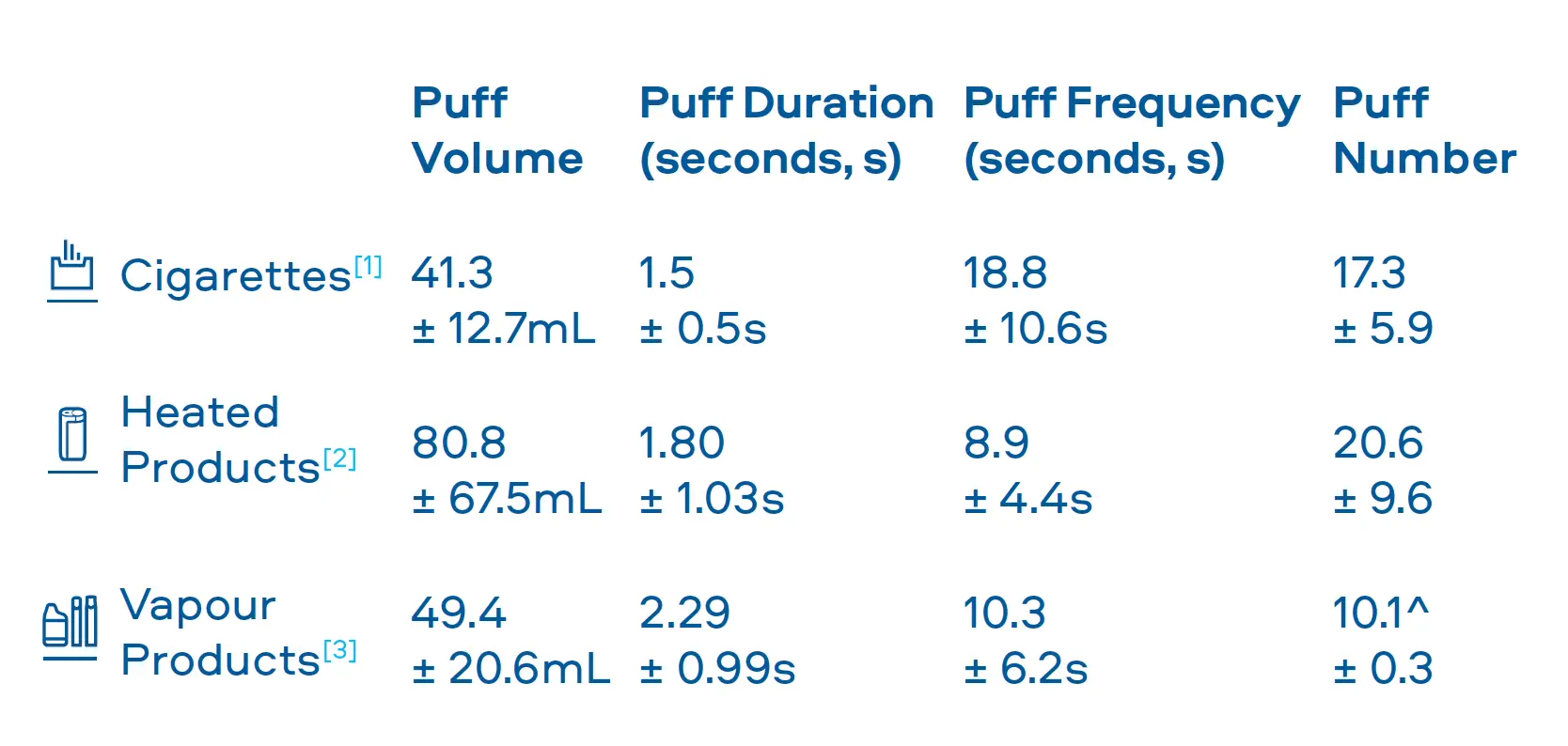
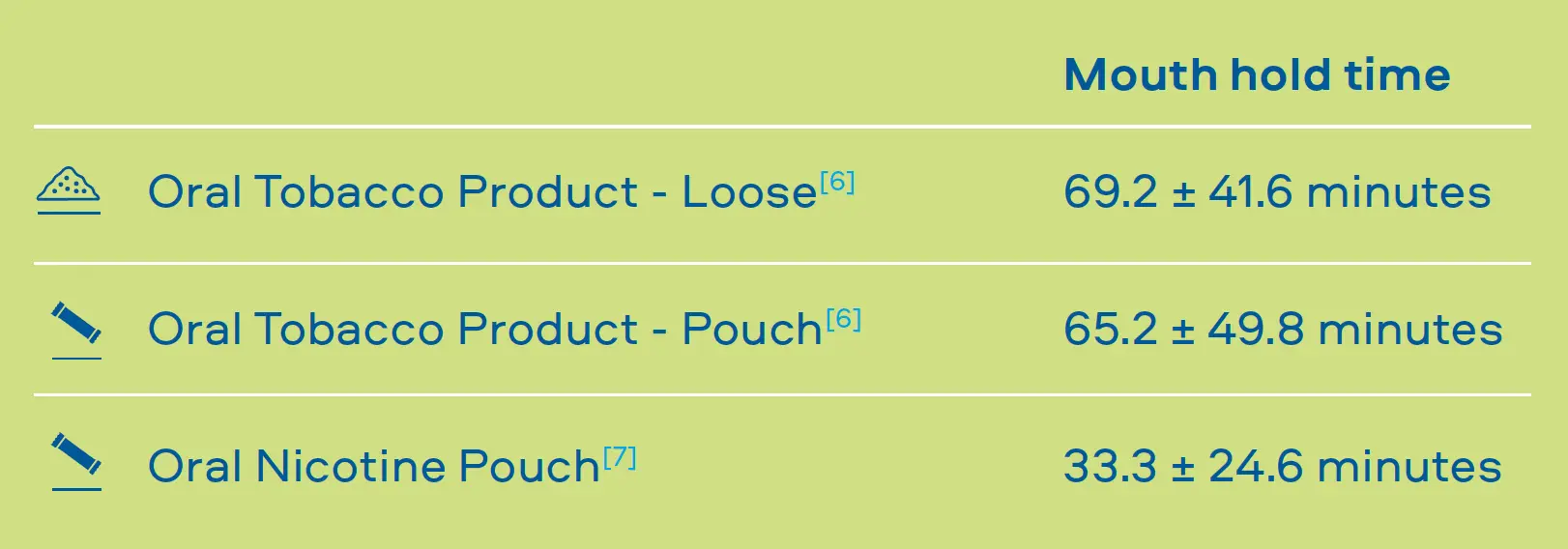
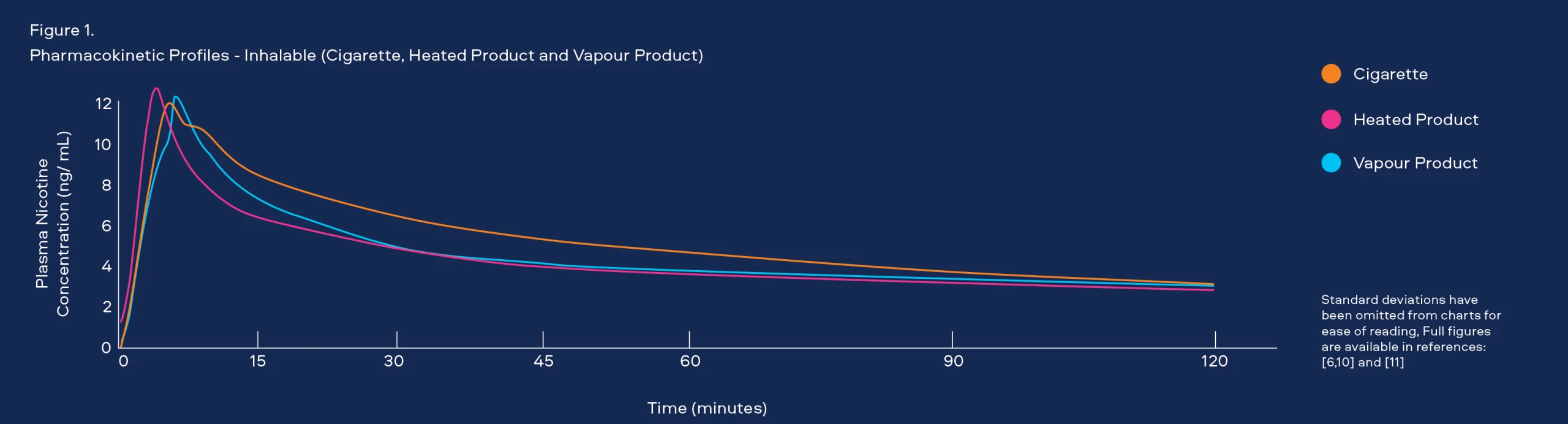
Through laboratory tests we understand that the emissions of our Smokeless Products are simpler than cigarette smoke and proven to be less toxic. However, it is important that we understand how adult consumers may use our products to confirm they do not use them in a manner that offsets these reductions. To understand consumer use behaviour we carry out studies that assess: